Vitamin C Vaccine
Introduction
Vitamin A deficiency (VAD) adversely affects children and adults worldwide. Today, the World Health Organization (WHO) estimates that 250 million preschool children suffer from VAD, with the highest frequencies among low-income areas of Africa and South-East Asia [http://www.who.int (accessed March 01, 2019)]. Infectious diseases, particularly respiratory and diarrheal diseases, occur at increased frequencies (at least 2:1) among populations with VAD compared to vitamin-replete populations (1).
The global burden of VAD and its effects on populations in developing countries are well known, but much less well appreciated are incidences of VAD and vitamin insufficiencies in the developed world (2–5). In Memphis, TN, we tested influenza virus-infected children and their household contacts for retinol binding protein (RBP) as a surrogate for vitamin A (6), and found that 13 of 21 individuals were either vitamin A insufficient or deficient (5). We found that both infected and uninfected study participants exhibited low RBP levels. Low RBP can be a consequence of illness (7), but also reflects conditions of malnutrition when individuals in low-income families have limited access to nutrient-rich foods (8–10). Whereas, infants in the United States may receive government-funded, vitamin-fortified formulas, comparable support is not given to older children (11). Instead, diets for older children and adults may be calorie-dense and nutrient-poor. As is the case in developing countries, vitamin insufficiencies and deficiencies in the developed world correlate with weakened immune responses and poor outcomes upon hospitalization for infectious disease (2, 4). Unlike the situation in the developing world, individuals in the United States are usually assumed to be vitamin A-replete. Malnutrition may therefore go unnoticed.
Vitamin A Requirements, Metabolism, and Trafficking
Vitamin A is acquired from the diet in the form of retinoids (preformed vitamin A) or carotenoids (provitamin A). Retinoids include retinol or retinyl esters from animal sources, whereas carotenoids include beta-carotenes from plants. The recommended daily allowance (RDA) for vitamin A is dependent on age and sex. The Office of Dietary supplements (ODS) at the National Institutes of Health (NIH) currently recommends an RDA ranging from 300 to 600 μg retinoic acid equivalents (RAE) for young children. For individuals aged ≥14 years, RDAs are 700 μg RAE for non-pregnant females and 900 μg RAE for males (1 IU retinol = 0.3 microgram RAE) [https://ods.od.nih.gov (accessed March 01, 2019)]. A blood level of <0.7 μM retinol is considered vitamin "deficient" or "inadequate," and levels between 0.7 and 1.05 μM retinol are considered "insufficient" or "marginal" for some biological functions (12).
Vitamin A is generally stored in the liver as esters, but can also be found in extra-hepatic sites such as lung, intestine, kidney, and adipose tissue (13, 14). Retinol is the most common vitamin A metabolite in the blood and typically circulates in a complex bound to RBP with a 1:1 molar ratio. Retinol-bound RBP (holo-RBP) is, in turn, often bound to transthyretin, a common serum transport protein (15, 16). Retinoids can also be transported by chylomicrons or chylomicron remnants in lymph and blood (14, 17). Intracellularly, retinol is converted by retinol dehydrogenases (RDH, ubiquitous enzymes) to retinal, and then by retinaldehyde dehydrogenases (RALDH, e.g., ALDH1A) in select tissues to retinoic acid (RA) (18–21). RA is the vitamin A metabolite best known for its ability to regulate innate and adaptive immune cell function, proliferation, and survival. Importantly, metabolism and trafficking of vitamin A, and consequent effects on the immune system, can be influenced by genetic backgrounds, diets, conditions of mal-adsorption, and obesity (22). In the case of obesity, animal experiments suggest that vitamin A may be deficient in tissues such as the lung, even when levels in blood appear to be replete (23).
Regulatory Functions of Vitamin A
Virtually every mammalian cell, including epithelial and immune cells, is affected by vitamin A (7, 24–34). Vitamin A is perhaps best known for its regulation of gene transcription. RA is a nuclear hormone that binds nuclear hormone receptors including the retinoic acid receptor (RAR) and the peroxisome proliferator-activated receptor (PPARβ/δ) (35, 36). Receptors, in turn, bind DNA and serve as transcription factors to enhance or inhibit gene expression (27). Multiple isoforms exist for RAR [e.g., RARα, β, and γ (37)], and each protein can bind to the retinoid X receptor (RXR) in a heterodimeric complex (27, 38).
Receptors are promiscuous in binding to their ligands and to DNA. The RAR-RXR heterodimer will often bind two half-site sequences, known as retinoic acid response elements (RAREs), separated by a short spacer in the DNA (27, 39–44). RAREs have a consensus sequence of 5′-(A/G)G(G/T)TCA-3′, though receptors can be bound to non-consensus DNA sites as well. The exact sequence and spacer length (typically zero to eight bases) can alter binding affinity. Additionally, receptors can bind indirectly to DNA by tethering to other DNA-bound factors. Cross-regulation between vitamin A and related nuclear hormones (e.g., vitamin D, thyroid hormone, or sex hormones) may occur, because nuclear hormone receptors can compete for binding to ligands, co-receptors, and DNA (27, 40, 45–48).
RAREs are found throughout the genome, often within gene promoters or enhancers. Notably, hotspots for RARE have been identified in switch sites of the immunoglobulin heavy chain locus, positions instrumental in class switch recombination (CSR) (49). The potential binding of nuclear hormone receptors to switch sites and regulatory elements in immunoglobulin and T cell receptor (TCR) loci predicts a direct mechanism by which vitamin A may modulate lymphocyte function (49–52).
Adding to the complexity of vitamin A functions are the extra-nuclear activities. Vitamins bind a complex array of escort proteins at the cell membrane and in extra-nuclear compartments. Each of these interactions can initiate or modulate cell signaling (53, 54).
Vitamin A and Immune Activities in vitro and in Small Animals
Essentially all cells of the immune system including innate cells, B cells, and T cells, are affected by vitamin A (31, 55–59). Research animals with VAD generate poor antibody responses to many pathogens including parainfluenza virus and influenza virus (34, 59–61). VAS, when administered either orally or intranasally, can correct responses when given at the time of vaccination (33, 59, 60, 62).
In vitro, vitamin A has been shown to upregulate IgA production by B cells (18, 63, 64), and skew T cell phenotypes toward Treg rather than Th17 populations (65–70), but in vivo, outcomes are less predictable (71). For example, whereas VAD cells may yield poor Treg activities in a tissue culture setting indicating a predisposition for heightened immune responses (65–68), Tregs are found at equal or greater frequencies in tissues of VAD mice compared to controls following a respiratory virus infection (32). Furthermore, VAD mice exhibit relatively poor pathogen-specific T cell responses in vivo. In studies of influenza virus and parainfluenza virus infections, there are only weak virus-specific CD8+ T cell responses in the lower respiratory tract (LRT) of VAD mice (26). Responding CD8+ T cells in VAD and vitamin A+D deficient (VAD+VDD) mice express high levels of membrane CD103 (the αE subunit of αEβ7, an e-cadherin receptor). Possibly, the poor recruitment of CD8+ T cells to the LRT is because LRT tissues express relatively low levels of e-cadherin, and CD103+ cells home preferentially to other sites (26). When VAD+VDD mice receive VAS (with or without supplemental vitamin D), CD103 levels on virus-specific CD8+ T cells are reduced, and the percentages of CD4+ and CD8+ T cells in the LRT are improved (72). As another example of the complex influences of vitamin A on immune responses, we find that serum antibody isotype distributions differ between VAD and control animals, but patterns are dependent on the animal's background and sex (50). As a last example, some studies show that VAD biases the immune response toward a Th1 profile and that high levels of vitamin A bias the response toward a Th2 profile (68, 73, 74). Nonetheless, outcomes are again dependent on cell targets, environment, and activation state (25). Both Th1 and Th2 cytokine responses are evident in VAD mice, and VAD animals express higher levels of Th1 and Th2 cytokines compared to controls at late stages following a respiratory virus infection, presumably as a consequence of poor virus clearance (32).
Vitamin A additionally influences epithelial cells and innate immune cells associated with mucosal surfaces. Dendritic cells of the intestine and epithelial cells of the respiratory tract each express the ALDH1A enzymes required for conversion of retinaldehyde to the end-metabolite RA (18, 26, 29). These unique attributes of mucosal tissues help explain why VAS assists immune responses when applied either orally or intranasally (33, 62).
Due to the plethora of immune cell and barrier cell requirements for vitamin A, it is not surprising that VAD associates with poor immune responses to vaccines, and that VAS can reverse these weaknesses when given at the time of vaccination (33, 34, 59–62). One vaccine that deserves continued study in the context of VAD and VAS is Prevnar-13. It is estimated that worldwide pneumococcus kills close to 1 million children under the age of 5 each year (75, 76). Prevnar-13 can protect against these mortalities (77, 78), but the vaccine-induced immune response is not always protective. We suggest that attention to, and correction of, low vitamin levels in Prevnar-13 vaccine recipients may improve vaccine success. Previous studies have shown that VAD inhibits responses both to individual pneumococcus antigens and to Prevnear-13 in mice (59, 61, 79–81). Here, we extend findings to show that VAS improves the immunogenicity and protective capacity of Prevnar-13 in VAD and control animals.
Materials and Methods
Mice and Vaccinations
Experiments were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at St. Jude Children's Research Hospital (St. Jude). St. Jude follows the standards of the Animal Welfare Act and the document entitled "Principles for the Use of Animals and Guide for the Care and Use of Laboratory Animals."
To produce VAD mice, pregnant C57BL/6 (H2-b) mice were purchased from Jackson Laboratories (Bar harbor, ME). Mice were placed on either a control or VAD diet upon their arrival in the animal facility at St. Jude (days 4–5 gestation). VAD (cat. no. 5WA2, Test Diets) and control (cat. no. 5W9M) diets differed only in vitamin A content, containing either 0 or 15 IU/g vitamin A palmitate, respectively. Mothers and progeny remained on their assigned diets. Experiments were begun when progeny reached adulthood. These adult mice were vaccinated with 2 doses of Prevnar-13 (PCV, Wyeth Pharmaceuticals Inc.) with 3 weeks intervals. Vaccine was diluted 1:40 in PBS and 100 μL of PCV was administered intraperitoneally (IP). Immediately prior to each vaccination, mice received either 600 IU vitamin A (from Interplexus Inc., Kent, WA) or PBS by oral gavage (100 μL).
Antibody Measurements by ELISA
Animals were bled 10–14 days after boosting. ELISA plates were coated with 50 μL/well of 5 μg/mL T4 polysaccharide (from American Type Culture Collection, ATCC, Manassas, VA) in PBS using an Integra Viaflo384 robot (Integra Biosciences, Hudson, NH) and incubated overnight at 4°C. Plates were then washed 3x with PBS using an Aquamax 4000 plate washer (Molecular Devices, San Jose, CA). Block was 1% BSA in PBS (200 μL/well) added robotically and incubated overnight at 4°C. Mouse serum samples were diluted 1:500 in dilution buffer (1% BSA + 0.05% Tween in PBS). Block was removed and samples were added to plates (50 μL/well) and incubated overnight at 4°C. Plates were then washed 3x with PBS +0.05% Tween using the plate washer. Developing antibodies were added robotically (100 μL/well). These were anti-mouse IgM (cat. no. 1020-04; Southern Biotech, Birmingham, AL), anti-mouse IgG1 (cat. no. 1070-04; Southern Biotech), or anti -mouse IgG3 (cat. no. 1100-04; Southern Biotech), each diluted 1:1000 in dilution buffer. Plates were incubated 1 h at room temperature and then washed 3x with PBS +0.05% Tween using the plate washer. Substrate (1 mg/mL of pNPP in diethanoloamine buffer; 100 μL/well) was added robotically to plates. Plates were developed for 5–15 min and read at 405 nm on a VersaMax Tunable Microplate Reader (Molecular Devices). Statistical comparisons were made using Mann Whitney tests and GraphPad Prism software (* p < 0.05, ** p < 0.01, *** p < 0.001).
Challenges Post-vaccination
To prepare bacteria for challenge experiments, S. pneumoniae strain TIGR4 (serotype 4) was inoculated from a glycerol stock onto a Tryptic Soy Agar plate (GranCult, Millipore, Burlington, MA) supplemented with 3% sheep blood (Lampire Biological Laboratory, Pipersville, PA) and 20 μg/mL neomycin, and grown at 37°C, 5% CO2. After overnight growth, bacteria were directly inoculated into Todd Hewitt broth (Becton Dickinson, BD, Sparks, MD) supplemented with 0.2% yeast extract (BD) and grown until mid-log phase, OD620 = 0.4. Cells were washed in PBS prior to animal infections.
To challenge mice, 2 weeks after the vaccine boost, animals were sedated with 3% isoflurane. They were then inoculated intranasally with 5 × 105 CFU S. pneumoniae in 100 μL PBS. To collect and titer lungs, 24 h after infections groups of animals were euthanized by CO2 asphyxiation and cervical dislocation. Lungs were removed, washed twice in ~1 mL of PBS and then placed in 0.5 mL PBS. Lungs were then pulverized with a mechanical tissue grinder. Following emulsification, lungs were spun for 5 min at 300 g to pellet debris. Supernatants from the lung homogenates were collected and serially diluted 1:10 in PBS five times. From each dilution, 10 μL were plated on a Tryptic Soy Agar plate (GranCult, Millipore) supplemented with 3% sheep blood (Lampire Biological Laboratory) and 20 μg/mL neomycin. Plates were incubated overnight at 37°C. Colonies were counted and Excel software was used to calculate titers. Separate groups of animals were infected as described above, monitored for signs of symptomatic infection, and euthanized when moribund.
Results
Vaccine studies were conducted with male and female mice (either VAD mice or vitamin-replete controls) that were given two successive IP immunizations, separated by 3 week intervals, with the Prevnar-13 vaccine. Mice received either 600 IU of vitamin A as retinyl palmitate by oral gavage or phosphate buffered saline (PBS) at the time of vaccination. Antibody responses were measured 10–14 days after the second vaccine dose. ELISAs were conducted to examine antibodies specific for the type 4 (T4) component of the vaccine. As shown in Figure 1, there was significant improvement of T4-specific antibodies, including IgM and IgG1 isotypes in VAD mice and IgG1 in control mice when VAS was used. IgG3 levels were not significantly changed. Results were reminiscent of previous studies in rats using bacterial antigens and retinol treatments (79–82).
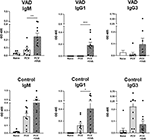
Figure 1. VAS and T4 polysaccharide-specific immune responses. Results from T4 ELISAs are shown for VAD (top row) and vitamin A-replete control (bottom row) mice. Separate ELISAs were conducted to measure T4-specific IgM IgG1, and IgG3 antibodies. Statistical comparisons were made using Mann Whitney tests and GraphPad Prism software (*p < 0.05, **p < 0.01, ***p < 0.001). IgM levels (for VAD mice), and IgG1 levels (for VAD and control mice), but not IgG3 levels, were significantly improved with VAS.
In a preliminary set of experiments, vaccinated animals were also challenged with a high-dose (5 × 105 colony forming units, CFU) of pneumococcus (Streptococcus pneumoniae strain TIGR4 [serotype 4]). At this dose, >90% of unvaccinated VAD and vitamin-replete control mice developed an infection, and the dose was 100% lethal in unvaccinated VAD animals (Figure 2). After 24 h, groups of 8–10 mice were sacrificed to measure lung titers. As shown, there were trends toward lower CFU in both VAD and control animals that received VAS at the time of vaccination. A separate set of mice were tested for survival post-challenge. There were significant improvements in survival for VAD and control animals that received VAS at the time of vaccination compared to unsupplemented, vaccinated animals. In fact, all animals that received VAS at the time of vaccination, regardless of original vitamin A status, survived.
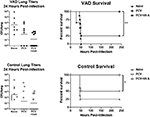
Figure 2. VAS with vaccination improves survival after challenge. Challenge results are shown for VAD (top row) and vitamin-replete control (bottom row) mice. CFU per lung were measured 24 h after challenge (left). In separate groups of mice, survival was monitored (right). Animals were sacrificed when moribund. Survival curves were compared using GraphPad Prism software (*p < 0.05, **p < 0.01).
Discussion
We have shown that VAS supports improvements in the immunogenicity of Prevnar-13 in VAD and control mice. With a preliminary study, we also showed that when VAS was given coincident with vaccination, protection against a subsequent challenge with S. pneumoniae was improved. Based on these promising results, we are now initiating a randomized clinical study to test the effects of VAS among Memphian children vaccinated with Prevnar-13 (clincaltrials.gov, PCVIT NCT03859687).
Previous Research on VAD and VAS in Humans
Public health organizations strive to improve dietary nutrition worldwide, but this goal depends on delivering vitamin-rich foods to all populations, a formidable task. For infants in countries where VAD is known to be endemic, the WHO has supported high-dose VAS programs (83). Children often receive 100,000 IU vitamin A between the ages of 6–11 months and 200,000 IU every 4–6 months between the ages of 12–59 months (84). Research analyses of VAS have yielded positive results in countries where VAD is frequent. In meta-analyses of clinical trials, VAS was shown to reduce deaths by 12–24%, and in isolated studies, reductions of 35–50% were observed (1, 85–92). VAS reduced morbidities due to infectious diseases, including measles, Plasmodium falciparum, and HIV (93–96). VAS benefits were also observed when antibody responses were measured, including those to vaccination (97, 98). Some studies have shown improved responses to the measles and tetanus toxoid (TT) vaccines following VAS (99–101).
Unfortunately, despite the positive influences described above, results from clinical VAS research have been inconsistent. VAS studies have often failed to show benefit, and have in some cases demonstrated risk. As an example, Malaba et al. did not observe an effect of VAS on infant mortality among children born to HIV-negative mothers with apparently adequate baseline vitamin A levels (102). There have also been reports of increased mother to child transmission (MTCT) of HIV in the context of VAS (103, 104). Additional noted risks of high-dose VAS were fontanelle bulging in infants (105, 106) and bone density loss [possibly due to cross-inhibition between related nuclear hormones, in this case vitamins A and D (107, 108)].
VAS studies in the context of vaccine programs have also yielded conflicting data (97, 109, 110). Brown et al. for example, showed no improvements by VAS on TT vaccinations (110) and a study of HIV-infected individuals showed no improvements by VAS on influenza virus vaccinations (111). A study by Semba et al. showed a negative influence of VAS on responses to the measles vaccine in 6 month old infants (112), unlike the situation for older infants (100, 113, 114). Explanations for differences in VAS efficacy among clinical studies have addressed effects of age, maternal antibodies, serum antibody levels, and serum vitamin levels, but a consensus has not been reached (90).
The contradictory results described above have encouraged the scientific community to question indiscriminate use of VAS, particularly in communities where nutrition has improved and where many children are vitamin replete (97, 106, 115). Suggestions are made to redirect efforts toward the use of low-dose VAS and/or toward support of improved diets (116).
One clear weakness in past clinical research is that comprehensive baseline vitamin levels of study participants for vitamin A and the related, cross-regulatory nuclear hormone vitamin D (47, 117–119) were rarely reported. Instead, vitamin status has often been predicted based on previous population studies (e.g., frequencies of xerophthalmia). This strategy does not address changing diets within communities or individual differences among study participants. Currently, perceptions of VAD frequencies may thus be falsely high for certain developing countries and falsely low for the developed world. The situation differs dramatically from research studies in small animals, wherein host backgrounds and diets are homogeneous and test animals differ from controls by a single defined variable. A full comprehension of how VAS differentially affects humans with replete, insufficient, or deficient vitamin A and D levels remains elusive.
A long-term solution to VAD in humans will require close attention to host characteristics, particularly baseline vitamin A and D levels (52). Improvements in diets should be a primary focus, with VAS programs developed as a back-up solution to malnutrition. For best outcomes with VAS, programs may require customization, with modification of supplements by frequency or dose, dependent on baseline characteristics of vaccine recipients. With attention to pre-existing vitamin levels and cautious administration, VAS programs may ultimately ensure that, (i) vaccinated children and adults are vitamin A replete worldwide, (ii) toxicities are avoided, and (iii) world populations maintain robust immune responses to pathogens and vaccines.
Data Availability
All datasets generated for this study are included in the manuscript.
Author Contributions
JH and JR contributed to experimental design, data analyses, and the writing and review of the manuscript. RP, HR, SS, and RS contributed to experimental design, performance of the experiments, data analyses, and the writing and review of the manuscript.
Funding
Funding was provided in part by NIH NCI P30 CA21765 and ALSAC.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Amy Iverson for assistance with experiments.
Abbreviations
RA, retinoic acid; RAR, retinoic acid receptor; RXR, retinoid-X receptor; PPAR, peroxisome proliferator-activated receptor; RARE, retinoic acid response element; VAD, vitamin A deficiency; VAS, vitamin A supplementation; CFU, colony forming units; AID, activation induced deaminase; RDA, recommended daily allowance; RAE, retinoic acid equivalents; RBP, retinol binding protein; WHO, world health organization; ODS, Office of Dietary Supplements; NIH, National Institutes of Health; CSR, class switch recombination; TCR, T cell receptor; IP, intraperitoneally.
References
1. Sommer A. Vitamin A, infectious disease, and childhood mortality: a 2 cent solution? J Infect Dis. (1993) 167:1003–7. doi: 10.1093/infdis/167.5.1003
CrossRef Full Text | Google Scholar
2. Stephens D, Jackson PL, Gutierrez Y. Subclinical vitamin A deficiency: a potentially unrecognized problem in the United States. Pediatr Nurs. (1996) 22:377–89.
PubMed Abstract | Google Scholar
3. Schall JI, Zemel BS, Kawchak DA, Ohene-Frempong K, Stallings VA. Vitamin A status, hospitalizations, and other outcomes in young children with sickle cell disease. J Pediatr. (2004) 145:99–106. doi: 10.1016/j.jpeds.2004.03.051
PubMed Abstract | CrossRef Full Text | Google Scholar
4. Hurwitz JL, Jones BG, Penkert RR, Gansebom S, Sun Y, Tang L, et al. Low retinol-binding protein and vitamin D levels are associated with severe outcomes in children hospitalized with lower respiratory tract infection and respiratory syncytial virus or human metapneumovirus detection. J Pediatr. (2017) 187:323–7. doi: 10.1016/j.jpeds.2017.04.061
CrossRef Full Text | Google Scholar
5. Jones BG, Oshansky CM, Bajracharya R, Tang L, Sun Y, Wong SS, et al. Retinol binding protein and vitamin D associations with serum antibody isotypes, serum influenza virus-specific neutralizing activities and airway cytokine profiles. Clin Exp Immunol. (2016) 183:239–47. doi: 10.1111/cei.12718
PubMed Abstract | CrossRef Full Text | Google Scholar
6. Almekinder J, Manda W, Soko D, Lan Y, Hoover DR, Semba RD. Evaluation of plasma retinol-binding protein as a surrogate measure for plasma retinol concentrations. Scand J Clin Lab Invest. (2000) 60:199–203. doi: 10.1080/003655100750044848
PubMed Abstract | CrossRef Full Text | Google Scholar
8. Mui Y, Lee BY, Adam A, Kharmats AY, Budd N, Nau C, et al. Healthy versus unhealthy suppliers in food desert neighborhoods: a network analysis of corner stores' food supplier networks. Int J Environ Res Public Health. (2015) 12:15058–74. doi: 10.3390/ijerph121214965
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Chaves GV, Peres WA, Gonçalves JC, Ramalho A. Vitamin A and retinol-binding protein deficiency among chronic liver disease patients. Nutrition. (2015) 31:664–8. doi: 10.1016/j.nut.2014.10.016
PubMed Abstract | CrossRef Full Text | Google Scholar
10. Quinlan KP, Hayani KC. Vitamin A and respiratory syncytial virus infection. Serum levels and supplementation trial. Arch Pediatr Adolesc Med. (1996) 150:25–30. doi: 10.1001/archpedi.1996.02170260029004
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Ellison RG, Greer BP, Burney JL, Goodell LS, Bower KB, Nicklas JC, et al. Observations and conversations: home preparation of infant formula among a sample of low-income mothers in the southeastern US. J Nutr Educ Behav. (2017) 49:579–87 e1. doi: 10.1016/j.jneb.2017.04.027
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Institute of Medicine Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. 2001. Washington, DC: National Academy Press.
Google Scholar
13. Nagy NE, Holven KB, Roos N, Senoo H, Kojima N, Norum KR, et al. Storage of vitamin A in extrahepatic stellate cells in normal rats. J Lipid Res. (1997) 38:645–58.
PubMed Abstract | Google Scholar
15. Zanotti G, Berni R. Plasma retinol-binding protein: structure and interactions with retinol, retinoids, and transthyretin. Vitam Horm. (2004) 69:271–95. doi: 10.1016/S0083-6729(04)69010-8
PubMed Abstract | CrossRef Full Text | Google Scholar
16. Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, et al. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J. (1999) 18:4633–44. doi: 10.1093/emboj/18.17.4633
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Quadro L, Hamberger L, Gottesman ME, Colantuoni V, Ramakrishnan R, Blaner WS. Transplacental delivery of retinoid: the role of retinol-binding protein and lipoprotein retinyl ester. Am J Physiol Endocrinol Metab. (2004) 286:E844–51. doi: 10.1152/ajpendo.00556.2003
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Rudraraju R, Jones BG, Surman SL, Sealy RE, Thomas PG, Hurwitz JL. Respiratory tract epithelial cells express retinaldehyde dehydrogenase ALDH1A and enhance IgA production by stimulated B cells in the presence of vitamin A. PLoS ONE. (2014) 9:e86554. doi: 10.1371/journal.pone.0086554
PubMed Abstract | CrossRef Full Text | Google Scholar
19. Wang C, Kane MA, Napoli JL. Multiple retinol and retinal dehydrogenases catalyze all-trans-retinoic acid biosynthesis in astrocytes. J Biol Chem. (2011) 286:6542–53. doi: 10.1074/jbc.M110.198382
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Sandell LL, Lynn ML, Inman KE, McDowell W, Trainor PA. RDH10 oxidation of Vitamin A is a critical control step in synthesis of retinoic acid during mouse embryogenesis. PLoS ONE. (2012) 7:e30698. doi: 10.1371/journal.pone.0030698
PubMed Abstract | CrossRef Full Text | Google Scholar
23. Ventura LL, Fortes NC, Santiago HC, Caliari MV, Gomes MA, Oliveira DR. Obesity leads to tissue, but not serum vitamin A deficiency. Sci Rep. (2015) 5:15893. doi: 10.1038/srep15893
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. (2004) 21:527–38. doi: 10.1016/j.immuni.2004.08.011
PubMed Abstract | CrossRef Full Text | Google Scholar
25. Penkert RR, Jones BG, Häcker H, Partridge JF, Hurwitz JL. Vitamin A differentially regulates cytokine expression in respiratory epithelial and macrophage cell lines. Cytokine. (2017) 91:1–5. doi: 10.1016/j.cyto.2016.11.015
PubMed Abstract | CrossRef Full Text | Google Scholar
26. Rudraraju R, Surman SL, Jones BG, Sealy R, Woodland DL, Hurwitz JL. Reduced frequencies and heightened CD103 expression among virus-induced CD8(+) T cells in the respiratory tract airways of vitamin A-deficient mice. Clin Vaccine Immunol. (2012) 19:757–65. doi: 10.1128/CVI.05576-11
PubMed Abstract | CrossRef Full Text | Google Scholar
28. Chopra DP, Klinger MM, Sullivan JK. Effects of vitamin A on growth and differentiation of human tracheobronchial epithelial cell cultures in serum-free medium. J Cell Sci. (1989) 93:133–42.
PubMed Abstract | Google Scholar
30. Mora JR, von Andrian UH. Retinoic acid: an educational "vitamin elixir" for gut-seeking T cells. Immunity. (2004) 21:458–60. doi: 10.1016/j.immuni.2004.10.002
CrossRef Full Text | Google Scholar
31. van de Pavert SA, Ferreira M, Domingues RG, Ribeiro H, Molenaar R, Moreira-Santos L, et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature. (2014) 508:123–7. doi: 10.1038/nature13158
PubMed Abstract | CrossRef Full Text | Google Scholar
32. Penkert RR, Surman SL, Jones BG, Sealy RE, Vogel P, Neale G, et al. Vitamin A deficient mice exhibit increased viral antigens and enhanced cytokine/chemokine production in nasal tissues following respiratory virus infection despite the presence of FoxP3+ T cells. Int Immunol. (2016) 28:139–52. doi: 10.1093/intimm/dxv064
PubMed Abstract | CrossRef Full Text | Google Scholar
33. Surman SL, Jones BG, Sealy RE, Rudraraju R, Hurwitz JL. Oral retinyl palmitate or retinoic acid corrects mucosal IgA responses toward an intranasal influenza virus vaccine in vitamin A deficient mice. Vaccine. (2014) 32:2521–4. doi: 10.1016/j.vaccine.2014.03.025
PubMed Abstract | CrossRef Full Text | Google Scholar
34. Surman SL, Rudraraju R, Sealy R, Jones B, Hurwitz JL. Vitamin A deficiency disrupts vaccine-induced antibody-forming cells and the balance of IgA/IgG isotypes in the upper and lower respiratory tract. Viral Immunol. (2012) 25:341–4. doi: 10.1089/vim.2012.0023
PubMed Abstract | CrossRef Full Text | Google Scholar
35. Shaw N, Elholm M, Noy N. Retinoic acid is a high affinity selective ligand for the peroxisome proliferator-activated receptor beta/delta. J Biol Chem. (2003) 278:41589–92. doi: 10.1074/jbc.C300368200
PubMed Abstract | CrossRef Full Text | Google Scholar
36. Choi JM, Bothwell AL. The nuclear receptor PPARs as important regulators of T-cell functions and autoimmune diseases. Mol Cells. (2012) 33:217–22. doi: 10.1007/s10059-012-2297-y
PubMed Abstract | CrossRef Full Text | Google Scholar
37. Ghyselinck NB, Dupé V, Dierich A, Messaddeq N, Garnier JM, Rochette-Egly C, et al. Role of the retinoic acid receptor beta (RARbeta) during mouse development. Int J Dev Biol. (1997) 41:425–47.
PubMed Abstract | Google Scholar
38. Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. (2007) 129:723–33. doi: 10.1016/j.cell.2007.02.050
PubMed Abstract | CrossRef Full Text | Google Scholar
39. Privalsky ML. The role of corepressors in transcriptional regulation by nuclear hormone receptors. Annu Rev Physiol. (2004) 66:315–360. doi: 10.1146/annurev.physiol.66.032802.155556
PubMed Abstract | CrossRef Full Text | Google Scholar
40. Lee S, Privalsky ML. Heterodimers of retinoic acid receptors and thyroid hormone receptors display unique combinatorial regulatory properties. Mol Endocrinol. (2005) 19:863–78. doi: 10.1210/me.2004-0210
PubMed Abstract | CrossRef Full Text | Google Scholar
41. Boergesen M, Pedersen TÅ, Gross B, van Heeringen SJ, Hagenbeek D, Bindesbøll C, et al. Genome-wide profiling of liver X receptor, retinoid X receptor, and peroxisome proliferator-activated receptor alpha in mouse liver reveals extensive sharing of binding sites. Mol Cell Biol. (2012) 32:852–67. doi: 10.1128/MCB.06175-11
PubMed Abstract | CrossRef Full Text | Google Scholar
42. Simons SS, Edwards DP, Kumar R. Minireview: dynamic structures of nuclear hormone receptors: new promises and challenges. Mol Endocrinol. (2014) 28:173–82. doi: 10.1210/me.2013-1334
PubMed Abstract | CrossRef Full Text | Google Scholar
44. Rochette-Egly C, Germain P. Dynamic and combinatorial control of gene expression by nuclear retinoic acid receptors (RARs). Nucl Recept Signal. (2009) 7:e005. doi: 10.1621/nrs.07005
PubMed Abstract | CrossRef Full Text | Google Scholar
45. Ide T, Shimano H, Yoshikawa T, Yahagi N, Amemiya-Kudo M, Matsuzaka T, et al. Cross-talk between peroxisome proliferator-activated receptor (PPAR) alpha and liver X receptor (LXR) in nutritional regulation of fatty acid metabolism. II. LXRs suppress lipid degradation gene promoters through inhibition of PPAR signaling. Mol Endocrinol. (2003) 17:1255–67. doi: 10.1210/me.2002-0191
CrossRef Full Text | Google Scholar
46. DiRenzo J, Söderstrom M, Kurokawa R, Ogliastro MH, Ricote M, Ingrey S, et al. Peroxisome proliferator-activated receptors and retinoic acid receptors differentially control the interactions of retinoid X receptor heterodimers with ligands, coactivators, and corepressors. Mol Cell Biol. (1997) 17:2166–76. doi: 10.1128/MCB.17.4.2166
PubMed Abstract | CrossRef Full Text | Google Scholar
47. Williams GR, Franklyn JA. Physiology of the steroid-thyroid hormone nuclear receptor superfamily. Baillieres Clin Endocrinol Metab. (1994) 8:241–66. doi: 10.1016/S0950-351X(05)80251-4
PubMed Abstract | CrossRef Full Text | Google Scholar
48. Winrow CJ, Capone JP, Rachubinski RA. Cross-talk between orphan nuclear hormone receptor RZRalpha and peroxisome proliferator-activated receptor alpha in regulation of the peroxisomal hydratase-dehydrogenase gene. J Biol Chem. (1998) 273:31442–8. doi: 10.1074/jbc.273.47.31442
PubMed Abstract | CrossRef Full Text | Google Scholar
49. Hurwitz JL, Penkert RR, Xu B, Fan Y, Partridge JF, Maul RW, et al. Hotspots for vitamin-steroid-thyroid hormone response elements within switch regions of immunoglobulin heavy chain loci predict a direct influence of vitamins and hormones on B cell class switch recombination. Viral Immunol. (2016) 29:132–6. doi: 10.1089/vim.2015.0104
PubMed Abstract | CrossRef Full Text | Google Scholar
50. Jones BG, Sealy RE, Penkert RR, Surman SL, Maul RW, Neale G, et al. Complex sex-biased antibody responses: estrogen receptors bind estrogen response elements centered within immunoglobulin heavy chain gene enhancers. Int Immunol. (2018) 31:141–56. doi: 10.1093/intimm/dxy074
PubMed Abstract | CrossRef Full Text | Google Scholar
51. Jones BG, Penkert RR, Xu B, Fan Y, Neale G, Gearhart PJ, et al. Binding of estrogen receptors to switch sites and regulatory elements in the immunoglobulin heavy chain locus of activated B cells suggests a direct influence of estrogen on antibody expression. Mol Immunol. (2016) 77:97–102. doi: 10.1016/j.molimm.2016.07.015
PubMed Abstract | CrossRef Full Text | Google Scholar
52. Sealy RE, Jones BG, Surman SL, Penkert RR, Pelletier S, Neale G, et al. Will attention by vaccine developers to the host's nuclear hormone levels and immunocompetence improve vaccine success? Vaccines. (2019) 7:E26. doi: 10.3390/vaccines7010026
PubMed Abstract | CrossRef Full Text | Google Scholar
53. Napoli JL. Cellular retinoid binding-proteins, CRBP, CRABP, FABP5: effects on retinoid metabolism, function and related diseases. Pharmacol Ther. (2017) 173:19–33. doi: 10.1016/j.pharmthera.2017.01.004
PubMed Abstract | CrossRef Full Text | Google Scholar
54. Kawaguchi R, Zhong M, Kassai M, Ter-Stepanian M, Sun H. Vitamin A transport mechanism of the multitransmembrane cell-surface receptor STRA6. Membranes. (2015) 5:425–53. doi: 10.3390/membranes5030425
PubMed Abstract | CrossRef Full Text | Google Scholar
57. Beijer MR, Molenaar R, Goverse G, Mebius RE, Kraal G, den Haan JM. A crucial role for retinoic acid in the development of Notch-dependent murine splenic CD8- CD4- and CD4+ dendritic cells. Eur J Immunol. (2013) 43:1608–16. doi: 10.1002/eji.201343325
PubMed Abstract | CrossRef Full Text | Google Scholar
58. Klebanoff CA, Spencer SP, Torabi-Parizi P, Grainger JR, Roychoudhuri R, Ji Y, et al. Retinoic acid controls the homeostasis of pre-cDC-derived splenic and intestinal dendritic cells. J Exp Med. (2013) 210:1961–76. doi: 10.1084/jem.20122508
PubMed Abstract | CrossRef Full Text | Google Scholar
59. Ross AC. Vitamin A supplementation and retinoic acid treatment in the regulation of antibody responses in vivo. Vitam Horm. (2007) 75:197–222. doi: 10.1016/S0083-6729(06)75008-7
PubMed Abstract | CrossRef Full Text | Google Scholar
60. Surman SL, Penkert RR, Jones BG, Sealy RE, Hurwitz JL. Vitamin supplementation at the time of immunization with a cold-adapted influenza virus vaccine corrects poor mucosal antibody responses in mice deficient for vitamins A and D. Clin Vaccine Immunol. (2016) 23:219–27. doi: 10.1128/CVI.00739-15
PubMed Abstract | CrossRef Full Text | Google Scholar
62. Surman SL, Jones BG, Rudraraju R, Sealy RE, Hurwitz JL. Intranasal administration of retinyl palmitate with a respiratory virus vaccine corrects impaired mucosal IgA response in the vitamin A-deficient host. Clin Vaccine Immunol. (2014) 21:598–601. doi: 10.1128/CVI.00757-13
PubMed Abstract | CrossRef Full Text | Google Scholar
63. Seo GY, Jang YS, Kim J, Choe J, Han HJ, Lee JM, et al. Retinoic acid acts as a selective human IgA switch factor. Hum Immunol. (2014) 75:923–9. doi: 10.1016/j.humimm.2014.06.021
PubMed Abstract | CrossRef Full Text | Google Scholar
64. Seo GY, Jang YS, Kim HA, Lee MR, Park MH, Park SR, et al. Retinoic acid, acting as a highly specific IgA isotype switch factor, cooperates with TGF-beta1 to enhance the overall IgA response. J Leukoc Biol. (2013) 94:325–35. doi: 10.1189/jlb.0313128
PubMed Abstract | CrossRef Full Text | Google Scholar
66. Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. (2007) 204:1775–85. doi: 10.1084/jem.20070602
PubMed Abstract | CrossRef Full Text | Google Scholar
67. Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. (2007) 204:1757–64. doi: 10.1084/jem.20070590
PubMed Abstract | CrossRef Full Text | Google Scholar
70. Tejón G, Manríquez V, De Calisto J, Flores-Santibáñez F, Hidalgo Y, Crisóstomo N, et al. Vitamin A impairs the reprogramming of Tregs into IL-17-producing cells during intestinal inflammation. Biomed Res Int. (2015) 2015:137893. doi: 10.1155/2015/137893
PubMed Abstract | CrossRef Full Text | Google Scholar
72. Surman SL, Jones BG, Woodland DL, Hurwitz JL. Enhanced CD103 expression and reduced frequencies of virus-specific CD8(+) T cells among airway lymphocytes after influenza vaccination of mice deficient in vitamins A + D. Viral Immunol. (2017) 30:737–43. doi: 10.1089/vim.2017.0086
PubMed Abstract | CrossRef Full Text | Google Scholar
73. Stephensen CB, Jiang X, Freytag T. Vitamin A deficiency increases the in vivo development of IL-10-positive Th2 cells and decreases development of Th1 cells in mice. J Nutr. (2004) 134:2660–6. doi: 10.1093/jn/134.10.2660
PubMed Abstract | CrossRef Full Text | Google Scholar
74. Spilianakis CG, Lee GR, Flavell RA. Twisting the Th1/Th2 immune response via the retinoid X receptor: lessons from a genetic approach. Eur J Immunol. (2005) 35:3400–4. doi: 10.1002/eji.200535588
PubMed Abstract | CrossRef Full Text | Google Scholar
75. Iroh Tam PY, Thielen BK, Obaro SK, Brearley AM, Kaizer AM, Chu H, et al. Childhood pneumococcal disease in Africa - A systematic review and meta-analysis of incidence, serotype distribution, and antimicrobial susceptibility. Vaccine. (2017) 35:1817–27. doi: 10.1016/j.vaccine.2017.02.045
PubMed Abstract | CrossRef Full Text | Google Scholar
76. O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. (2009) 374:893–902. doi: 10.1016/S0140-6736(09)61204-6
PubMed Abstract | CrossRef Full Text | Google Scholar
77. Boelsen LK, Dunne EM, Lamb KE, Bright K, Cheung YB, Tikoduadua L, et al. Long-term impact of pneumococcal polysaccharide vaccination on nasopharyngeal carriage in children previously vaccinated with various pneumococcal conjugate vaccine regimes. Vaccine. (2015) 33:5708–14. doi: 10.1016/j.vaccine.2015.07.059
PubMed Abstract | CrossRef Full Text | Google Scholar
78. Greenhow TL, Hung YY, Herz A. Bacteremia in children 3 to 36 months old after introduction of conjugated pneumococcal vaccines. Pediatrics. (2017) 139:e20162098. doi: 10.1542/peds.2016-2098
PubMed Abstract | CrossRef Full Text | Google Scholar
79. Pasatiempo AM, Kinoshita M, Taylor CE, Ross AC. Antibody production in vitamin A-depleted rats is impaired after immunization with bacterial polysaccharide or protein antigens. FASEB J. (1990) 4:2518–27. doi: 10.1096/fasebj.4.8.2110538
CrossRef Full Text | Google Scholar
80. Pasatiempo AM, Bowman TA, Taylor CE, Ross AC. Vitamin A depletion and repletion: effects on antibody response to the capsular polysaccharide of Streptococcus pneumoniae, type III (SSS-III). Am J Clin Nutr. (1989) 49:501–10. doi: 10.1093/ajcn/49.3.501
PubMed Abstract | CrossRef Full Text | Google Scholar
81. Pasatiempo AM, Taylor CE, Ross AC. Vitamin A status and the immune response to pneumococcal polysaccharide: effects of age and early stages of retinol deficiency in rats. J Nutr. (1991) 121:556–62. doi: 10.1093/jn/121.4.556
PubMed Abstract | CrossRef Full Text | Google Scholar
82. Ross AC. Vitamin A deficiency and retinoid repletion regulate the antibody response to bacterial antigens and the maintenance of natural killer cells. Clin Immunol Immunopathol. (1996) 80:S63–72. doi: 10.1006/clin.1996.0143
PubMed Abstract | CrossRef Full Text | Google Scholar
83. Irlam JH, Visser MM, Rollins NN, Siegfried N. Micronutrient supplementation in children and adults with HIV infection. Cochrane Database Syst Rev. (2010) CD003650. doi: 10.1002/14651858.CD003650.pub3
CrossRef Full Text | Google Scholar
84. Vitamin A supplementation: who when and how. Community Eye Health. (2013) 26:71.
85. Humphrey JH, Agoestina T, Wu L, Usman A, Nurachim M, Subardja D, et al. Impact of neonatal vitamin A supplementation on infant morbidity and mortality. J Pediatr. (1996) 128:489–96. doi: 10.1016/S0022-3476(96)70359-1
PubMed Abstract | CrossRef Full Text | Google Scholar
86. Nacul LC, Kirkwood BR, Arthur P, Morris SS, Magalhães M, Fink MC. Randomised, double blind, placebo controlled clinical trial of efficacy of vitamin A treatment in non-measles childhood pneumonia. BMJ. (1997) 315:505–10. doi: 10.1136/bmj.315.7107.505
PubMed Abstract | CrossRef Full Text | Google Scholar
87. Imdad A, Mayo-Wilson E, Herzer K, Bhutta ZA. Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age. Cochrane Database Syst Rev. (2017) 3:CD008524. doi: 10.1002/14651858.CD008524.pub3
PubMed Abstract | CrossRef Full Text | Google Scholar
88. Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ. (2011) 343:d5094. doi: 10.1136/bmj.d5094
PubMed Abstract | CrossRef Full Text | Google Scholar
89. Sommer A, Tarwotjo I, Hussaini G, Susanto D. Increased mortality in children with mild vitamin A deficiency. Lancet. (1983) 2:585–8. doi: 10.1016/S0140-6736(83)90677-3
PubMed Abstract | CrossRef Full Text
91. Vitamin A supplementation in northern Ghana: effects on clinic attendances hospital admissions and child mortality. Ghana VAST Study Team. Lancet. (1993) 342:7–12. doi: 10.1016/0140-6736(93)91879-Q
CrossRef Full Text
92. West KP, Pokhrel RP, Katz J, LeClerq SC, Khatry SK, Shrestha SR, et al. Efficacy of vitamin A in reducing preschool child mortality in Nepal. Lancet. (1991) 338:67–71. doi: 10.1016/0140-6736(91)90070-6
PubMed Abstract | CrossRef Full Text | Google Scholar
93. D'Souza RM, D'Souza R. Vitamin A for treating measles in children. Cochrane Database Syst Rev. (2001) CD001479.
Google Scholar
94. Humphrey JH, Iliff PJ, Marinda ET, Mutasa K, Moulton LH, Chidawanyika H, et al. Effects of a single large dose of vitamin A, given during the postpartum period to HIV-positive women and their infants, on child HIV infection, HIV-free survival, and mortality. J Infect Dis. (2006) 193:860–71. doi: 10.1086/500366
PubMed Abstract | CrossRef Full Text | Google Scholar
95. Fawzi WW, Mbise RL, Hertzmark E, Fataki MR, Herrera MG, Ndossi G, et al. A randomized trial of vitamin A supplements in relation to mortality among human immunodeficiency virus-infected and uninfected children in Tanzania. Pediatr Infect Dis J. (1999) 18:127–33. doi: 10.1097/00006454-199902000-00009
PubMed Abstract | CrossRef Full Text | Google Scholar
96. Shankar AH, Genton B, Semba RD, Baisor M, Paino J, Tamja S, et al. Effect of vitamin A supplementation on morbidity due to Plasmodium falciparum in young children in Papua New Guinea: a randomised trial. Lancet. (1999) 354:203–9. doi: 10.1016/S0140-6736(98)08293-2
PubMed Abstract | CrossRef Full Text | Google Scholar
97. Villamor E, Fawzi WW. Effects of vitamin a supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev. (2005) 18:446–64. doi: 10.1128/CMR.18.3.446-464.2005
PubMed Abstract | CrossRef Full Text | Google Scholar
98. Rahman MM, Mahalanabis D, Hossain S, Wahed MA, Alvarez JO, Siber GR, et al. Simultaneous vitamin A administration at routine immunization contact enhances antibody response to diphtheria vaccine in infants younger than six months. J Nutr. (1999) 129:2192–5. doi: 10.1093/jn/129.12.2192
PubMed Abstract | CrossRef Full Text | Google Scholar
99. Semba RD, Muhilal Scott AL, Natadisastra G, Wirasasmita S, Mele L, et al. Depressed immune response to tetanus in children with vitamin A deficiency. J Nutr. (1992) 122:101–7. doi: 10.1093/jn/122.1.101
PubMed Abstract | CrossRef Full Text | Google Scholar
100. Benn CS, Aaby P, Balé C, Olsen J, Michaelsen KF, George E, et al. Randomised trial of effect of vitamin A supplementation on antibody response to measles vaccine in Guinea-Bissau, west Africa. Lancet. (1997) 350:101–5. doi: 10.1016/S0140-6736(96)12019-5
PubMed Abstract | CrossRef Full Text | Google Scholar
102. Malaba LC, Iliff PJ, Nathoo KJ, Marinda E, Moulton LH, Zijenah LS, et al. Effect of postpartum maternal or neonatal vitamin A supplementation on infant mortality among infants born to HIV-negative mothers in Zimbabwe. Am J Clin Nutr. (2005) 81:454–60. doi: 10.1093/ajcn.81.2.454
PubMed Abstract | CrossRef Full Text | Google Scholar
104. Fawzi WW, Msamanga GI, Hunter D, Renjifo B, Antelman G, Bang H, et al. Randomized trial of vitamin supplements in relation to transmission of HIV-1 through breastfeeding and early child mortality. AIDS. (2002) 16:1935–44. doi: 10.1097/00002030-200209270-00011
PubMed Abstract | CrossRef Full Text | Google Scholar
105. de Francisco A, Chakraborty J, Chowdhury HR, Yunus M, Baqui AH, Siddique AK, et al. Acute toxicity of vitamin A given with vaccines in infancy. Lancet. (1993) 342:526–7. doi: 10.1016/0140-6736(93)91648-6
PubMed Abstract | CrossRef Full Text | Google Scholar
106. Vijayaraghavan K, Radhaiah G, Prakasam BS, Sarma KV, Reddy V. Effect of massive dose vitamin A on morbidity and mortality in Indian children. Lancet. (1990) 336:1342–5. doi: 10.1016/0140-6736(90)92895-O
PubMed Abstract | CrossRef Full Text | Google Scholar
108. Melhus H, Michaëlsson K, Kindmark A, Bergström R, Holmberg L, Mallmin H, et al. Excessive dietary intake of vitamin A is associated with reduced bone mineral density and increased risk for hip fracture. Ann Intern Med. (1998) 129:770–8. doi: 10.7326/0003-4819-129-10-199811150-00003
PubMed Abstract | CrossRef Full Text | Google Scholar
109. Kutukculer N, Akil T, Egemen A, Kurugöl Z, Akşit S, Ozmen D, et al. Adequate immune response to tetanus toxoid and failure of vitamin A and E supplementation to enhance antibody response in healthy children. Vaccine. (2000) 18:2979–84. doi: 10.1016/S0264-410X(00)00097-9
PubMed Abstract | CrossRef Full Text | Google Scholar
110. Brown KH, Rajan MM, Chakraborty J, Aziz KM. Failure of a large dose of vitamin A to enhance the antibody response to tetanus toxoid in children. Am J Clin Nutr. (1980) 33:212–7. doi: 10.1093/ajcn/33.2.212
PubMed Abstract | CrossRef Full Text | Google Scholar
111. Hanekom WA, Yogev R, Heald LM, Edwards KM, Hussey GD, Chadwick EG. Effect of vitamin A therapy on serologic responses and viral load changes after influenza vaccination in children infected with the human immunodeficiency virus. J Pediatr. (2000) 136:550–2. doi: 10.1016/S0022-3476(00)90024-6
PubMed Abstract | CrossRef Full Text | Google Scholar
112. Semba RD, Munasir Z, Beeler J, Akib A, Muhilal Audet S, et al. Reduced seroconversion to measles in infants given vitamin A with measles vaccination. Lancet. (1995) 345:1330–2. doi: 10.1016/S0140-6736(95)92536-8
PubMed Abstract | CrossRef Full Text | Google Scholar
113. Semba RD, Akib A, Beeler J, Munasir Z, Permaesih D, Muherdiyantiningsih, et al. Effect of vitamin A supplementation on measles vaccination in nine-month-old infants. Public Health. (1997) 111:245–7. doi: 10.1038/sj.ph.1900366
PubMed Abstract | CrossRef Full Text | Google Scholar
114. Benn CS, Balde A, George E, Kidd M, Whittle H, Lisse IM, et al. Effect of vitamin A supplementation on measles-specific antibody levels in Guinea-Bissau. Lancet. (2002) 359:1313–4. doi: 10.1016/S0140-6736(02)08274-0
PubMed Abstract | CrossRef Full Text | Google Scholar
115. Bhattacharya S, Singh A. Phasing out of the universal mega dose of vitamin-A prophylaxis to avoid toxicity. AIMS Public Health. (2017) 4:38–46. doi: 10.3934/publichealth.2017.1.38
PubMed Abstract | CrossRef Full Text | Google Scholar
116. Rahmathullah L, Underwood BA, Thulasiraj RD, Milton RC, Ramaswamy K, Rahmathullah R, et al. Reduced mortality among children in southern India receiving a small weekly dose of vitamin A. N Engl J Med. (1990) 323:929–35. doi: 10.1056/NEJM199010043231401
PubMed Abstract | CrossRef Full Text | Google Scholar
117. Beildeck ME, Gelmann EP, Byers SW. Cross-regulation of signaling pathways: an example of nuclear hormone receptors and the canonical Wnt pathway. Exp Cell Res. (2010) 316:1763–72. doi: 10.1016/j.yexcr.2010.02.001
PubMed Abstract | CrossRef Full Text | Google Scholar
118. Tavera-Mendoza L, Wang TT, Lallemant B, Zhang R, Nagai Y, Bourdeau V, et al. Convergence of vitamin D and retinoic acid signalling at a common hormone response element. EMBO Rep. (2006) 7:180–5. doi: 10.1038/sj.embor.7400594
PubMed Abstract | CrossRef Full Text | Google Scholar
119. Cao X, Teitelbaum SL, Zhu HJ, Zhang L, Feng X, Ross FP. Competition for a unique response element mediates retinoic acid inhibition of vitamin D3-stimulated transcription. J Biol Chem. (1996) 271:20650–4. doi: 10.1074/jbc.271.34.20650
PubMed Abstract | CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fimmu.2019.01576/full

0 Komentar